so hmmm first off, let's use the decimal format of a percentage, thus, 6% is rather just 6/100 or 0.06, and 15% is just 15/100 or just 0.15 and so on
now

whatever "x" and "y" may be, we know they must add up to 100 ounces, thus
x + y = 100
and whatever the concentrated amounts in the solution are for each, they must add up to 10 oz, thus
0.06x + 0.15y = 10
thus
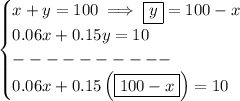
solve for "x", to see how much of the 6% solution will be needed
what about "y"? well, y = 100 - x