Answer: The correct answer is- It is an exothermic reaction because energy is lost.
As per the question, the starting energy of reactants for cellular respiration is 3564 kilojoules and energy of products is 2878 kilojoules.
We can find out whether a reaction is exothermic or endothermic by calculating ΔH that is energy change or enthalpy change of a reaction.
A negative value of ΔH represents exothermic reaction ( energy is lost) whereas a positive value represents endothermic reaction ( energy is consumed).
ΔH =
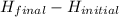
ΔH = 2878- 3564
ΔH = -686 kilojoules
As the value of ΔH is negative, this represents energy is lost in this reaction and therefore, it is an exothermic process.