Answer: Option (a) is the correct answer.
Step-by-step explanation:
A chemical reaction in which there will be absorption of heat energy by the reactant molecules is known as endothermic reaction.
For example,
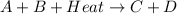 is an endothermic reaction.
is an endothermic reaction.
On the other hand, a chemical reaction in which there will be release of heat energy is known as an exothermic reaction.
For example,
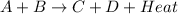 is an exothermic reaction.
is an exothermic reaction.
Therefore, we can conclude that the given thermochemical equation
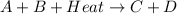 shows endothermic type of reaction.
shows endothermic type of reaction.