Answer: Option (B) is the correct answer.
Step-by-step explanation:
Radioactive reaction for given carbon atom is as follows.
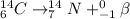
It is known that in a beta decay, a neutron tends to convert into a proton along with emission of a high energy electron which is ejected from the nucleus of the atom in the form of beta particle.
Basically, a beta decay occurs when there are many neutrons present inside a nucleus as compared to the protons.
Also, when there is more difference in the ratio or number of protons and neutrons then nucleus of the atom becomes unstable in nature. This unstability is caused due to greater repulsion between the like charges of sub-atomic particles.
As a result, this force of repulsion becomes greater than the binding energy. And, this force is known as the weak force because it is unable to bind the neutrons and protons together.
Thus, we can conclude that a fundamental force which allows this conversion to occur is a neutron is converted into a proton; weak force.