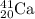Answer: Calcium
Step-by-step explanation: As the element contains 20 electrons and 20 protons, it contains equal number of positive and negative charges, and thus it is a neutral atom.
Atomic number of an element = number of protons = number of electrons = 20
Thus the element is Ca with atomic number 20.
Mass number of an element = number of electrons + number of neutrons= 20+21= 41
Thus the representation of the element is:
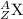
Where X = chemical symbol
A = mass number
Z = atomic number
Thus the representation is