Answer:
30 kJ is released during the combustion of this sample.
Step-by-step explanation:
Heat released during combustion = Heat gained by calorimeter
Mass of calorimeter= m = 1.00 kg=1000 g
Change in temperature of the calorimeter = ΔT = 41.0 °C - 21.0 °C =20.0 °C
Specific heat if calorimeter = c = 1.50 J/g °C
Heat released during combustion = Q
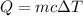
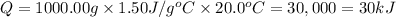
30 kJ is released during the combustion of this sample.