Answer : The enthalpy change for this reaction is, -414.49 KJ
Solution :
The balanced chemical reaction is,
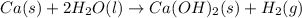
The expression for enthalpy change is,
![\Delta H_(rxn)=\sum [n* \Delta H_f(product)]-\sum [n* \Delta H_f(reactant)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/68pjxk6shkewh9hcm4y8smnbicjb2jeayo.png)
![\Delta H_(rxn)=[(n_(H_2)* \Delta H_(H_2))+(n_(Ca(OH)_2)* \Delta H_(Ca(OH)_2))]-[(n_(Ca)* \Delta H_(Ca))+(n_(H_2O)* \Delta H_(H_2O))]](https://img.qammunity.org/2018/formulas/chemistry/high-school/ykd7zbxwdh96dxb5irq4dgom16nwc7lufl.png)
where,
n = number of moles
As we know that standard enthalpy of an element and standard gas always be zero.
![\Delta H_(rxn)=[(n_(Ca(OH)_2)* \Delta H_(Ca(OH)_2))]-[(n_(H_2O)* \Delta H_(H_2O))]](https://img.qammunity.org/2018/formulas/chemistry/high-school/ee3o7bx8nmz8yw1i2qm52oxl6gpflhyh4x.png)
Now put all the given values in this expression, we get
![\Delta H_(rxn)=[(1mole* -986.09KJ/mole)]-[(2mole* -285.8KJ/mole)]\\\\\Delta H_(rxn)=-414.49KJ](https://img.qammunity.org/2018/formulas/chemistry/high-school/z7prgc4e3cfaq1j9ra9arfdnqlr2o3lxbx.png)
Therefore, the enthalpy change for this reaction is, -414.49 KJ