Answer : The
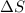 system for this change is, negative
system for this change is, negative
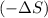 .
.
Explanation :
Entropy : It measure the randomness and the disorderedness of the system.
As we know that the entropy increases,
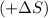 as we are moving from the solid state to liquid state and liquid state to gaseous state and the entropy decreases,
as we are moving from the solid state to liquid state and liquid state to gaseous state and the entropy decreases,
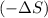 as we are moving from gaseous state to liquid state and liquid state to solid state.
as we are moving from gaseous state to liquid state and liquid state to solid state.
The given reaction is,
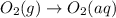
In this reaction, the phase changes from gaseous state to liquid or aqueous state. That means the entropy of the system for this change will be negative.
Hence, the
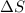 system for this change is, negative
system for this change is, negative
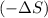 .
.