Answer: Choice B is correct.
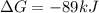 and
and
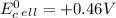
Explanation:
 stands for Gibbs free energy equation. A reaction is spontaneous if
stands for Gibbs free energy equation. A reaction is spontaneous if
 is negative and the reaction non spontaneous if
is negative and the reaction non spontaneous if
 is positive.
is positive.
For a cell,
 is calculated from the cell potential using the equation:
is calculated from the cell potential using the equation:
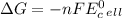
where, n is the moles of electrons transferred in a balanced equation, F is the faraday constant and
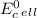 is standard cell potential.
is standard cell potential.
From the above equation,
 could only be negative if
could only be negative if
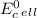 is positive as the equation has negative sign.
is positive as the equation has negative sign.
So, the only correct choice is the one for which
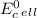 is positive and
is positive and
 is negative.
is negative.
Hence. choice B is correct.