Step-by-step explanation:
According to Arrhenius, acids are the species that readily dissociate into hydrogen or hydronium ions when dissolved in water.
For example,
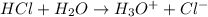
On the other hand, Arrhenius bases are the species that readily dissociate to give hydroxide ions in water.
For example,
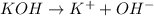
Hence, we can conclude that Arrhenius acid is the term that gives the behavior of substance that is dissolved in water and produces hydronium ions.