Answer:
![K_(sp)=[Cu^+]^2[CO_3^(2-)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/ymaj5e6lc2zfhekahsci9otau508d8ie77.png)
Step-by-step explanation:
Solubility product is defined as the equilibrium constant in which a solid ionic compound is dissolved to produce its ions in solution. It is represented as
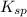
The equation for the ionization of the given compound is given as:
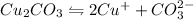
By stoichiometry of the reaction:
1 mole of
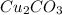 gives 2 moles of
gives 2 moles of
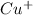 and 1 mole of
and 1 mole of
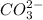 .
.
When the solubility of
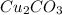 is S moles/liter, then the solubility of
is S moles/liter, then the solubility of
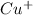 will be 2S moles\liter and solubility of
will be 2S moles\liter and solubility of
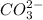 will be S moles/liter.
will be S moles/liter.
Expression for the equilibrium constant of
 will be:
will be:
![K_(sp)=[Cu^+]^2[CO_3^(2-)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/ymaj5e6lc2zfhekahsci9otau508d8ie77.png)