Answer: The correct option are(A),(B) and (D).
Step-by-step explanation:
Single Displacement reaction is define as chemical reaction in which more reactive element displaces the less reactive element from its compound. It is generally given as:
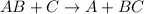
Reactions in option (A) and (B) represents single displacement reaction.:
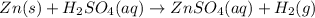
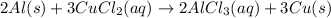
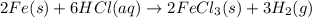
Hence, the correct option are(A) ,(B)and (D).