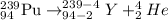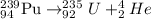Answer: D)
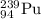
Explanation:
Alpha decay : When a larger nuclei decays into smaller nuclei by releasing alpha particle. In this process, the mass number and atomic number is reduced by 4 and 2 units respectively.
General representation of alpha decay :
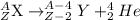
Thus