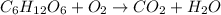Answer:substitution
Step-by-step explanation:
Addition reaction is defined as the reaction in which two reactants get added to give single product.
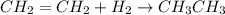
Substitution reaction is defined as the reaction in which one atom replaces the other atom from the compound.
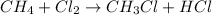
Hydrogenation is addition of hydrogen to unsaturated hydrocarbons.
![CH_2=CH_2+H_2\xrightarrow[]{hydrogenation} CH_3CH_3](https://img.qammunity.org/2018/formulas/chemistry/high-school/g9jazohsbhve8ieumhyz5cky089xhogv55.png)
Combustion is a type of chemical reaction in which fuel is reacted with oxygen to form carbon dioxide and water.
Example: