Answer:
1) The correct answer is option A.
2) The correct answer is option A.
Step-by-step explanation:
1) Electrolytes are the substance which break down into ions when dissolved in water and thus from current conducting liquid solutions .
These compounds in solid form does not conduct electricity due to the absence of free ions.
Magnesium chloride dissociate into its constituent ions when dissolved in water.
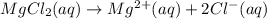
Magnesium chloride is the correct answer.
2) Molarity of a 785 ml solution that contains 6.5 moles of HCl.
Volume of solution = 785 mL = 0.785 L
Moles of HCl = 6.5 mol
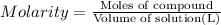
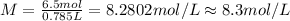
Hence, the correct answer is option A.