Answer: Option (a) is the correct answer.
Step-by-step explanation:
A solution which has more number of hydrogen ions will be acidic in nature and its pH will be less than 7. Whereas a solution which has more number of hydroxide ions will be basic in nature and thus, its pH will be greater than 7.
Also, pH =
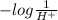
Therefore, a low pH means pH will be very less than 7.
The given molecules will dissociate as follows.
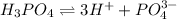
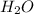 being a neutral molecule will not dissociate and pH of water is 7. Hence, it is a neutral molecule.
being a neutral molecule will not dissociate and pH of water is 7. Hence, it is a neutral molecule.
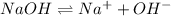
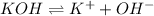
So, we can see that
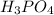 gives maximum number of hydrogen ions. Therefore, we can conclude that molecule of
gives maximum number of hydrogen ions. Therefore, we can conclude that molecule of
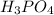 will form a solution with a very low pH.
will form a solution with a very low pH.