Answer:The nitrogen gas will diffuse fastest at a given temperature.
Step-by-step explanation:
Graham duhem law: 'The rate of diffusion of gas is inversely proportional to the square root of the molar mass of the gas'.
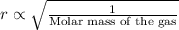
So, the gas with lowest molar mass will diffuse fastest at given temperature:
Mass of chlorine gas = 70 g .mol
Mass of fluorine gas = 38 g/mol
Mass of Argon gas = 39.95 g/mol
Mass of nitrogen gas = 28 g/mol

The nitrogen gas will diffuse fastest at a given temperature.