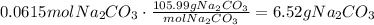The first step to solve this question is to find how many moles of HCl we need to neutralize. To do it, multiply the volume of solution times the concentration of HCl:
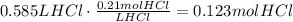
According to the given equation, 2 moles of HCl react with 1 mole of Na2CO3. The next step is to use this ratio to find the amount of Na2CO3 that reacts with 0.123 moles of HCl:
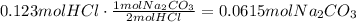
The final step is to use the molar mass of Na2CO3 to find the mass that reacts to neutralize the acid: