Answer: 80.442 grams
Explanation:
We now that the formula to calculate density is given by :-
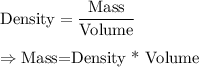
Given : Volume = 163.5 milliliter
Density = 0.492 grams/milliliter
Now, the weight (in grams) of a liquid is given by :-
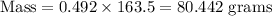
Hence, the weight of liquid = 80.442 grams