Answer: a concentrated solution
Explanation: A stock solution is a concentrated solution which is used to prepare solutions of lower concentrations by diluting it with addition of water.
But on diluting the number of moles remain same and thus we can use molarity equation.
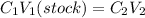 (to be prepared)
(to be prepared)
where,
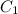 =concentration of stock solution
=concentration of stock solution
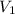 = volume of stock solution
= volume of stock solution
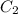 = concentration of solution to be prepared
= concentration of solution to be prepared
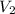 = volume of solution to be prepared
= volume of solution to be prepared