Answer: The elements present in Group 13 acquire a charge of +3 when they form ions.
Step-by-step explanation:
An ion is formed when a neutral atom looses or gains electrons.
- When an atom looses electrons, it results in the formation of positive ion known as cation.
- When an atom gains electrons, it results in the formation of negative ion known as anion.
The elements present in Group 13 of the periodic table are Boron (B), Aluminium (Al), Gallium *Ga), Indium (In) and Thallium (Tl)
General electronic configuration of the elements present in Group 13 is:
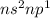
These elements have a tendency to loose all of the 3 electrons and form
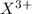 ions
ions
Hence, the elements present in Group 13 acquire a charge of +3 when they form ions.