Answer : The correct option is, (a) just as many electrons as protons.
Explanation :
For the neutral atom, the number of protons and electrons are equal. But, they are unequal when the atoms present in the form of ions or the atom has some charges.
When an unequal number of electrons and protons then it leads to the formation of ionic species.
Ion : An ion is formed when an atom looses or gains electron.
When an atom looses electrons, it will form a positive ion known as cation.
When an atom gains electrons, it will form a negative ion known as anion.
For example : The neutral atom K has equal number of protons and electrons i.e 19 but
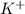 ion has unequal number of protons and electrons that means it has 19 number of protons and 18 number of electrons.
ion has unequal number of protons and electrons that means it has 19 number of protons and 18 number of electrons.
Hence, the correct option is, (a) just as many electrons as protons.