Answer : The correct option is, 0.888 g
Solution : Given,
As we know that the radioactive decays follow first order kinetics.
First we have to calculate the rate constant of a radioisotope.
Formula used :
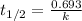
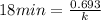
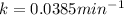
Now we have to calculate the amount remains after 72 minutes.
The expression for rate law for first order kinetics is given by :
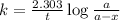
where,
k = rate constant =
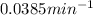
t = time taken for decay process = 72 min
a = initial amount of the reactant = 14.2 g
a - x = amount left after decay process = ?
Putting values in above equation, we get the value of amount left.
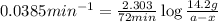
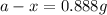
Therefore, the amount remain after 72 min will be, 0.888 g