Answer: 6
Step-by-step explanation:
This is a type of radioactive decay and all the radioactive process follow first order kinetics.
Half life is the time taken for an radioactive substance to decompose to half of its original value.
Now, to calculate the number of half lives, we use the formula:
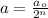
where,
a = amount of reactant left after 6 minutes =
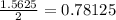
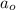 = Initial amount of the reactant = 50
= Initial amount of the reactant = 50
n = number of half lives = ?
Putting values in above equation, we get:
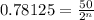
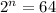
Taking log on both sides, we get
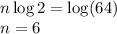
Thus 6 half lives occur during 6 minutes.