Answer: High activation energy is required.
Step-by-step explanation:
Unsaturated hydrocarbons are defined as the hydrocarbons in which double or triple bonds are present between two adjacent carbon atoms. They are known as alkenes and alkynes respectively. The general formula for these hydrocarbons is
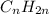 and
and
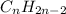
In unsaturated hydrocarbons, more number of bonds are formed, thus the bond strength of the bonds formed will be more because the orbitals come closer to each other.
As, bond strength of unsaturated hydrocarbons are more. So, more energy will be required to break the bond between them.
Hence, high activation energy is required.