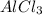Answer: The chemical formula of a compound formed between aluminium and chlorine atom will be
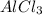
Step-by-step explanation:
Aluminium is the 13th element of the periodic table with electronic configuration of
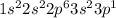
As, this element will easily loose 3 electrons and hence will show an oxidation state of +3.
Chlorine is the 17th element of the periodic table with electronic configuration
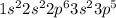
This element will easily gain 1 electron and shows an oxidation state of -1.
By criss-cross method, the oxidation state of aluminium and chlorine gets exchanged and thus they form the coefficients of the compound.
The compound formed by the combination of aluminium and chlorine will be
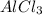
Hence, the chemical formula for the compound will be