Answer: The amount of air that could be held in lungs is 6.873g
Step-by-step explanation:
To calculate the amount of air, we use the equation given by Ideal gas equation, which follows:
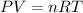
where,
P = Pressure of the gas = 102 kPa
V = volume of the gas = 6 L
n = number of moles of gas = ? mol
R = Gas constant =
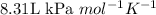
T = temperature of the gas = 37°C = 310 K (Conversion factor:
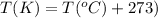
Putting values in above equation, we get:
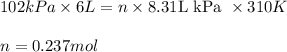
To calcultae the mass of the air, we use the equation:
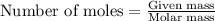
We are given:
Moles of air entered = 0.237 mol
Molar mass of air = 29 g/mol
Putting values in above equation, we get:
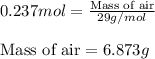
Hence, the amount of air that could be held in lungs is 6.873g