Step-by-step explanation:
Atomic number of lithium is 3 with electronic distribution 2, 1. So, to attain stability lithium loses 1 electron and becomes
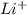 ion.
ion.
Atomic number of oxygen is 8 with electronic distribution 2, 6. So, to attain stability oxygen needs to gain two electrons and thus becomes
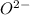 ion.
ion.
Therefore, a neutral molecule will have charges balanced between the combining atoms.
Hence, when lithium and oxygen atoms combine together then they will form compound
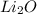 .
.