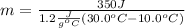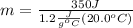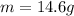Answer:
Step-by-step explanation:
You can use the equation
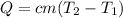 where Q is the heat, c is the specific heat of the substance,
where Q is the heat, c is the specific heat of the substance,
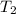 and
and
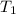 are temperature and m is the mass of the substance.
are temperature and m is the mass of the substance.
Solving for m we have:
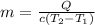
The problem gives you the information about the variables:
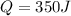
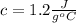
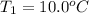
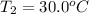
Replacing that values: