Answer:
1.0 mol of A should be left over.
Step-by-step explanation:
We have the balanced equation:
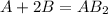
That means that I need 1.0 mol of A for each 2.0 moles of B to obtain 1.0 mol of
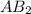
Now, we have 3.0 moles of A and 4.0 moles of B reacting, so:
3.0 moles of A .
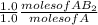 = 3.0 moles of
= 3.0 moles of
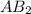
4.0 moles of B .
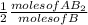 = 2.0 moles of
= 2.0 moles of
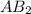
That means that with 3.0 moles of A and 4.0 moles of B, we can form 2.0 moles of
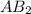 and 1.0 mole of A should be left over.
and 1.0 mole of A should be left over.