So this is nomenclature.
The name would be Iron (III) Chloride Hexahydrate
Reason why it's Iron (III) is because of the 3 after Cl, if you take FeCl3 apart it's
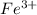
and Cl^-.
Hexahydrate because hexa = 6 and hydrate=water
The term hydrate would be wrong because that clearly doesn't come first
It would be the anhydrous compound that comes first. The "an" in "anhydrous" means "without". Hydrous=water, so anyhdrous= without water
The part without water is FeCl3. The water is 6H2O
The prefix for the coefficient of water is 6, or hexa, but that's not first in the name