Answer: The Correct option is A.
Explanation: Empirical formula of a molecule is defined as the simplest whole number ratio of atoms present in that molecule.
So, for a molecule having formula
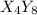 , the simplest whole number ratio becomes when we divide the stoichiometry by 4, which makes up the formula as
, the simplest whole number ratio becomes when we divide the stoichiometry by 4, which makes up the formula as
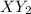 .
.
Therefore, the correct option is A.