Answer: low activation energy
Explanation:
Activation energy is the extra energy required by the reactants to overcome the energy barrier to convert to products.
Unsaturated hydrocarbons are defined as the hydrocarbons which have double or triple covalent C-C bonds. They are known as alkenes and alkynes respectively. The general formula for these hydrocarbons is
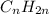 and
and
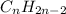 .
.
Unsaturated hydrocarbons are more reactive than the saturated ones because of the presence of
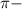 bonds. They are weaker than
bonds. They are weaker than
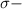 bonds and thus can be easily broken and thus require less energy for conversion into products.
bonds and thus can be easily broken and thus require less energy for conversion into products.