To balance the equation we start by counting the number of atoms we have of each element on each side of the reaction.
Reagents side:
Fe = 1 atom
Cl= 3 atoms
Na=1 atom
O=1 atom
H= 1atom
Products side:
Fe = 1 atom
Cl= 1 atom
Na=1 atom
O= 3 atoms
H= 3 atoms
Let's start with chlorine, we have 3 chlorine atoms on the reactant side and 1 on the product side. So we will put the coefficient 3 in the NaCl molecule to balance the chlorine.
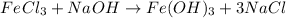
Now, we will have:
Reagents side:
Fe = 1 atom
Cl= 3 atoms
Na=1 atom
O=1 atom
H= 1atom
Products side:
Fe = 1 atom
Cl= 3 atom
Na= 3 atom
O= 3 atoms
H= 3 atoms
Now, we balance the Na, we have 1 atom of Na in the reactants and 3 in the products. We then put coefficient 3 in the NaOH molecule.
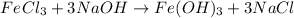
We have now the equation balanced:
Reagents side:
Fe = 1 atom
Cl= 3 atoms
Na=3 atoms
O=3 atoms
H= 3 atoms
Products side:
Fe = 1 atom
Cl= 3 atom
Na= 3 atom
O= 3 atoms
H= 3 atoms
We have the same number of atoms of each element on both sides of the reaction.
Now, in the reaction we can notice that Cl and OH happen to substitute the place of the other, therefore, this reaction will be a double replacement reaction.