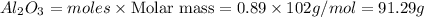Answer: 91.29 grams
Step-by-step explanation:
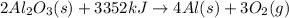
Endothermic reactions are those chemical reactions in which heat is absorbed by the reactants.
According to stoichiometry,
3352 kJ of energy is absorbed by 2 moles of
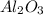
Thus 1500 kJ of energy will be absorbed by=
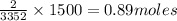 of
of
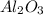
Mass of