The correct answer to the question is lead i.e the daughter element is lead.
Step-by-step explanation:
Before going to answer this question, first we have to understand alpha decay.
During alpha decay from a unstable massive radioactive substance, alpha particle will be emitted.
An alpha particle is a doubly charged helium nucleus. Hence, during alpha decay, the atomic number of the parent nuclei will be reduced by two and mass by four i.e the daughter nuclei will be placed two places left to the parent nuclei in the periodic table.
In the given question, we have Polonium- 218.
The atomic number of polonium is 84.
Hence, the atomic number of daughter nuclei will be 84 - 2= 82 .
The element which has atomic number 82 is lead ( Pb ).
The alpha decay of polonium is given as below -
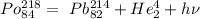 .
.
Here,
 is the frequency of the emitted radiations.
is the frequency of the emitted radiations.