Answer:
32.894 seconds ≈ 33 seconds
Explanation:
Function :
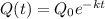
The function models radioactive decay of krypton-91.
The decay constant k is approximately 0.07.
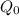 denotes initial amount .
denotes initial amount .
We are require to find How long will it take a quantity of krypton-91 to decay to 10% of its original amount
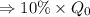
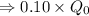
So, we neet to find t at which
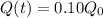
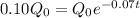
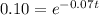
Taking log both sides
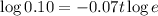
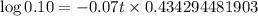
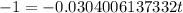
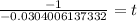
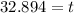
Thus it will take 32.894 seconds ≈ 33 seconds a quantity of krypton-91 to decay to 10% of its original amount.