Answer: 3248.0 kPa of pressure will be exerted by 10 moles of hydrogen gas in a 7.5 L cylinder at 20°C.
Explanation:
Using ideal gas equation:
PV = nRT
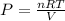
P= pressure = ?
V= volume =
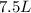
n = no of moles = 10 moles
R= gas constant =0.0821 kPaL\molK
T= temperature =
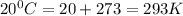
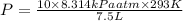
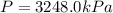
Thus pressure exerted is 3248.0 kPa