Answer:
Option d. f sublevel
Step-by-step explanation:
Inner transition elements includes:
Lanthanides
Actinides
Lanthanides includes elements having atomic number ranges from 52-71.
Actinides includes elements having atomic number ranges from 89-103.
Actinides and lanthanides have been placed separately in two rows below all the other elements.
General electronic configuration of lanthanides:
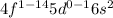
General electronic configuration of actinides:
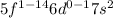
As last electrons enters in f sub shell or sublevel, these elements are also called f-block elements.