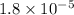Answer:
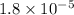
Step-by-step explanation:
Concentration of
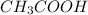 =
=
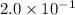
Concentration of
 =
=
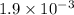
Concentration of
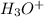 =
=
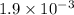
The balanced equilibrium reaction will be,
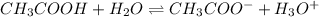
The expression for equilibrium reaction will be,
![K=([H_3O^+]* [CH_3COO^-])/([CH_3COOH])](https://img.qammunity.org/2018/formulas/chemistry/high-school/8rp4rawl0v449ivmev7x3cun02x1hbswzi.png)
Now put all the given values in this expression
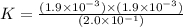
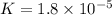
Therefore, the value of Keq for this reaction is