Answer:
D. The speed of molecules
Step-by-step explanation:
The temperature of a substance is directly related to the speed of the random motion of the molecules inside the substance: the faster the molecules move, the higher the temperature of the substance.
This relationship can be even quantified. For instance, for an ideal monoatomic gas, the average kinetic energy of the atoms inside the gas (
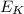 ) is related to the absolute temperature of the gas (T) by the equation
) is related to the absolute temperature of the gas (T) by the equation

where k is the Boltzmann constant. However, we know that the kinetic energy is related to the average speed of the molecules by
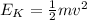
where v is the speed: therefore, the temperature is an indirect measurement of the speed of the molecules.