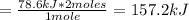Answer:
Molar heat of vaporization of ethanol, 157.2 kJ/mol
Step-by-step explanation:
Molar heat of vaporization is the amount heat required to vaporize 1 mole of a liquid to vapor.
The equilibrium is represented as:
C2H5OH(l) ↔ C2H5OH(g)
Given:
Mass of ethanol = 92.0 g
Energy required = 78.6 kJ
Calculation:
Molar mass of ethanol = 46 g/mol
Moles of ethanol =
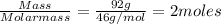
78.6 kJ of energy is required to vaporise 2 moles of ethanol
Therefore, the amount of energy required per mole would be: