Answer:
The water allows it to absorb large amounts of heat energy and only slightly increase in temperature because water's high specific heat.
(B) is correct option.
Step-by-step explanation:
Heat energy :
Heat energy is the product of mass of substance, specific heat capacity of substance and change in temperature.
In mathematically term,
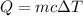
Where, m = mass of substance
 change in temperature
change in temperature
c = specific heat capacity
Specific heat capacity :
The amount of heat absorbed by 1 mole of a substance to raise its temperature by 1° C.
Water has the high specific heat.
The specific heat of water is 1 cal/g°C = 4.18 J/g°C.
Hence, The water allows it to absorb large amounts of heat energy and only slightly increase in temperature because water's high specific heat.