Answer : Option D)
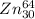
Explanation : When a positron is getting absorbed it means it will be
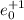 so, the
so, the
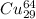 will get converted;
will get converted;
So, the whole reaction will be;
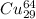 +
+
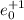 ---->
---->
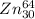 .
.
This will convert the whole element of Cu will get changed into Zn. As, it absorbs by the positron, the atomic number gets increased from 29 to 30.