Answer: 292.95 J
Step-by-step explanation:
change in internal energy= Heat transfer - work done
ΔU =Q -PΔV
Here, Q = 0 as there is no heat transfer.
P =2.00 atm = 2.00 × 101235 Pa = 202470 Pa
ΔV = final volume - initial volume = 0.8 V -V = -0.2 V
where V is the initial volume.
Volume of a spherical ball,
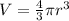
r = d/2 = 23.9 cm / 2 = 0.12 m
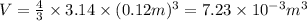
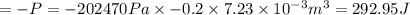
Hence, internal energy would change by 292.95 J.