Answer:
A inner d orbital complex must be proposed.
Step-by-step explanation:
Hello,
It is seen that in this complex the total charge is +3 as long as the
 is neutral. Therefore, its electron configuration is:
is neutral. Therefore, its electron configuration is:
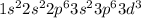
It means that it has three electrons on 3d sublevel zero on 4s and zero on 4p. Thus, the hybridization comes up from the fact we've got to complete the hollow spaces (six as there are six waters) into a whole "inner d orbital complex" that is:
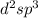
Best regards.