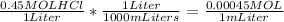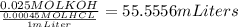Answer:
55.55 mL of HCl 0.45 m are needed to neutralize 25 mL of 1 m KOH
Step-by-step explanation:
The equation that describes the reaction is:
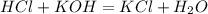
We can see that one mole of HCl is needed to consume one mole of KOH
Looking at the units:
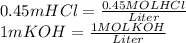
So, kepping in mind the previous statements, we proceed to calculate the total moles of KOH in the solution:
Now we know that we need 0.025 MOL of HCl to neutralize the solution; So: