This is an incomplete question, here is a complete question.
The radioactive isotope
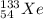 is used in pulmonary respiratory studies to image the blood flow and the air reaching the lungs. The half-life of this isotope is 5 days .
is used in pulmonary respiratory studies to image the blood flow and the air reaching the lungs. The half-life of this isotope is 5 days .
How long a time t will it take for the
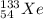 to decay so that eventually its activity decreases by a factor of 1024?
to decay so that eventually its activity decreases by a factor of 1024?
Express your answer numerically in days.
Answer : The time passed in days is, 50 days
Explanation :
Half-life = 5 days
First we have to calculate the rate constant, we use the formula :
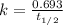
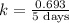
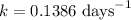
Now we have to calculate the time passed.
Expression for rate law for first order kinetics is given by:
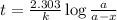
where,
k = rate constant =
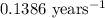
t = time passed by the sample = ?
a = initial amount of the reactant = 1
a - x = amount left after decay process =
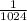
Now put all the given values in above equation, we get
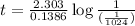
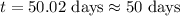
Therefore, the time passed in days is, 50 days