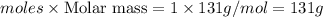Answer: 131 grams
Explanation:-
According to avogadro's law, 1 mole of every substance occupies 22.4 Liters at STP and contains avogadro's number
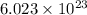 of particles.
of particles.
To calculate the moles, we use the equation:
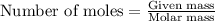
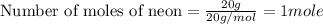
Thus 1 mole of Xenon will occupy the same volume as 1 mole of Neon.
Mass of xenon=