Answer:
Nickel (II) to solid nickel.
Step-by-step explanation:
Hello,
In this case, the placed species are nickel (II) sulfate
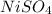 , zinc sulfate
, zinc sulfate
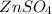 and solid iron
and solid iron
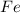 . Now, as the nickel sulfate is more reactive than the zinc sulfate, it will experience the following reduction half-reaction from nickel (II) to solid nickel:
. Now, as the nickel sulfate is more reactive than the zinc sulfate, it will experience the following reduction half-reaction from nickel (II) to solid nickel:
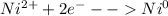
Nevertheless, it is possible that the zinc might experience the same reduction reaction from zinc (II) to solid zinc as well.
Best regards.